Chapter 6 Outcome Modeling
This chapter covers covariate-adjusted regression models that test associations between individual protein abundance levels and outcomes, including both continuous and binary responses. In contrast to differential abundance analysis covered in Chapters 4 and 5, where protein abundance (NPQ) was modeled as the outcome, in this setting single-protein NPQ serves as the primary predictor of interest, and outcomes such as disease status or a continuous clinical score is the dependent variable.
Note on terminology: These are univariable regression models (one protein at a time), not multivariable predictive or machine learning approaches. We use “outcome modeling” to describe fitting statistical models that quantify protein-outcome relationships while adjusting for relevant covariates.
6.1 Overview
While differential abundance asks “What proteins differ between groups?”, predictive modeling explores “Which proteins are associated with an outcome, and how well does protein abundance explain or predict it?”
In this context, “prediction” does not necessarily imply clinical forecasting. It refers more generally to building statistical models that quantify the relationship between protein abundance and a phenotype or outcome. These models use protein abundance level as a predictor to identify proteins associated with biological variation or group classification.
NULISAseqR provides four functions for outcome modeling:
Fixed Effects Models:
lmNULISAseq_predict(): For continuous outcomes (linear regression)glmNULISAseq_predict(): For binary or count outcomes (generalized linear models, including logistic, Poisson, etc.)
Mixed Effects Models:
lmerNULISAseq_predict(): For continuous outcomes with repeated measures or hierarchical data (linear mixed-effects models)glmerNULISAseq_predict(): For binary or count outcomes with repeated measures or hierarchical data (generalized linear mixed-effects models)
In this chapter, we will focus on the fixed effects models: lmNULISAseq_predict() for continuous outcomes and glmNULISAseq_predict() for binary outcomes.
6.2 Example 1: Exploring Age Associations (Continuous Outcome)
Age is a great continuous outcome for regression modeling because it is objectively measured, precisely recorded, and biologically meaningful — many protein abundance patterns naturally vary with aging.
6.2.1 Why Model Age?
- Identify proteins associated with aging
- Explore potential biological age biomarkers
- Understand how protein abundance relates to age
- Validate that your proteomic data captures true biological signal
6.2.2 Age Distribution in the Dataset
# Check age distribution in the Detectability Study dataset used in differential abundance analysis
summary(metadata$age)#> Min. 1st Qu. Median Mean 3rd Qu. Max. NA's
#> 22.00 42.75 55.00 54.66 65.25 86.00 19
The dataset contains a few pooled plasma samples with missing age data. We will exclude these samples from the age association analysis.
# Exclude samples with missing age
metadata_age <- metadata %>% filter(!is.na(age))
sample_list_age <- metadata_age$SampleName
cat("Samples with valid age:", nrow(metadata_age), "\n")#> Samples with valid age: 1486.2.4 Understanding the Age Model
For each protein, the model is:
age ~ NPQ + disease_type + sexThis tests: “Is this protein associated with age, while accounting for disease type and sex?”
The coefficient shows the estimated change in age per unit increase (doubling) in protein abundance (NPQ).
6.2.5 Examining Age Association Results
#> [1] "modelStats"#> Preview of Age Association Results (rounded to 3 digits):Key Columns in Results
target: Protein nametarget_coef: Estimated change in years of age per unit increase in NPQtarget_pval_unadj: Unadjusted p-value (is this protein significantly associated with age?)target_pval_FDR: FDR-adjusted p-valuetarget_pval_bonf: Bonferroni-adjusted p-value
6.2.6 Finding Age-Associated Proteins
We will identify proteins that are significantly associated with age with FDR-adjusted p value < 0.05.
# Find proteins significantly associated with age
age_proteins <- lmpt_age$modelStats %>%
filter(target_pval_FDR < 0.05) %>%
arrange(target_pval_FDR) %>%
select(target, target_coef, target_pval_FDR)
cat("Number of proteins significantly associated with age:", nrow(age_proteins), "\n\n")#> Number of proteins significantly associated with age: 8#> Age-Associated Proteins:
We can further identify proteins that are either significantly increased or decreased with age.
# Proteins that increase with age (positive coefficients)
age_increase <- age_proteins %>%
filter(target_coef > 0) %>%
arrange(desc(abs(target_coef)))
# Proteins that decrease with age (negative coefficients)
age_decrease <- age_proteins %>%
filter(target_coef < 0) %>%
arrange(desc(abs(target_coef)))
cat("Number of proteins significantly increase with age:", nrow(age_increase), "\n\n")#> Number of proteins significantly increase with age: 8#> Number of proteins significantly decrease with age: 0
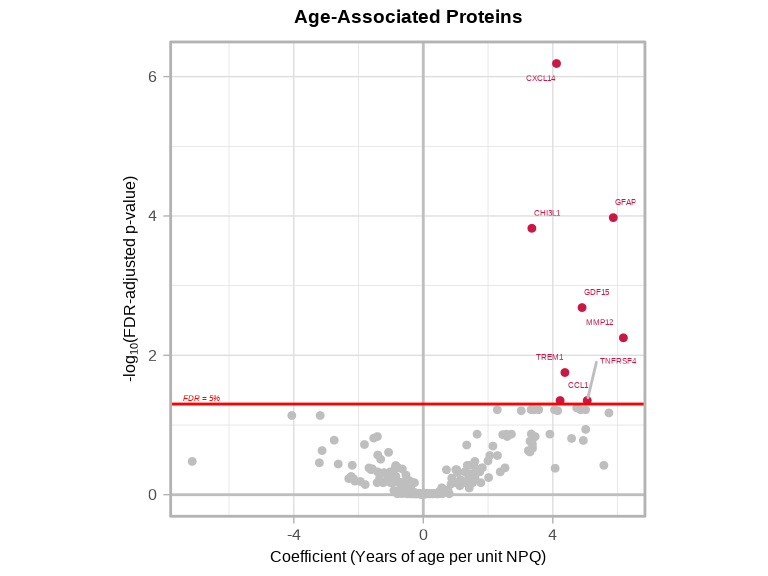
Interpretation:
- GFAP has
target_coef = 5.76, this means that every 1-unit increase in NPQ (2-fold increase in GFAP) is associated with an average age increase of 5.76 years - GDF15 has
target_coef = 4.25, this means that every 1-unit increase in NPQ (2-fold increase in GDF15) is associated with an average age increase of 4.25 years - CXCL14 has
target_coef = 3.47, this means that every 1-unit increase in NPQ (2-fold increase in CXCL14) is associated with an average age increase of 3.47 years - CHI3L1 has
target_coef = 3.33, this means that every 1-unit increase in NPQ (2-fold increase in CHI3L1) is associated with an average age increase of 3.33 years
6.2.7 Volcano Plots
volcanoPlot(
coefs = lmpt_age$modelStats$target_coef,
p_vals = lmpt_age$modelStats$target_pval_FDR,
target_labels = lmpt_age$modelStats$target,
title = "Age-Associated Proteins",
xlab = "Coefficient (Years of age per unit NPQ)",
)
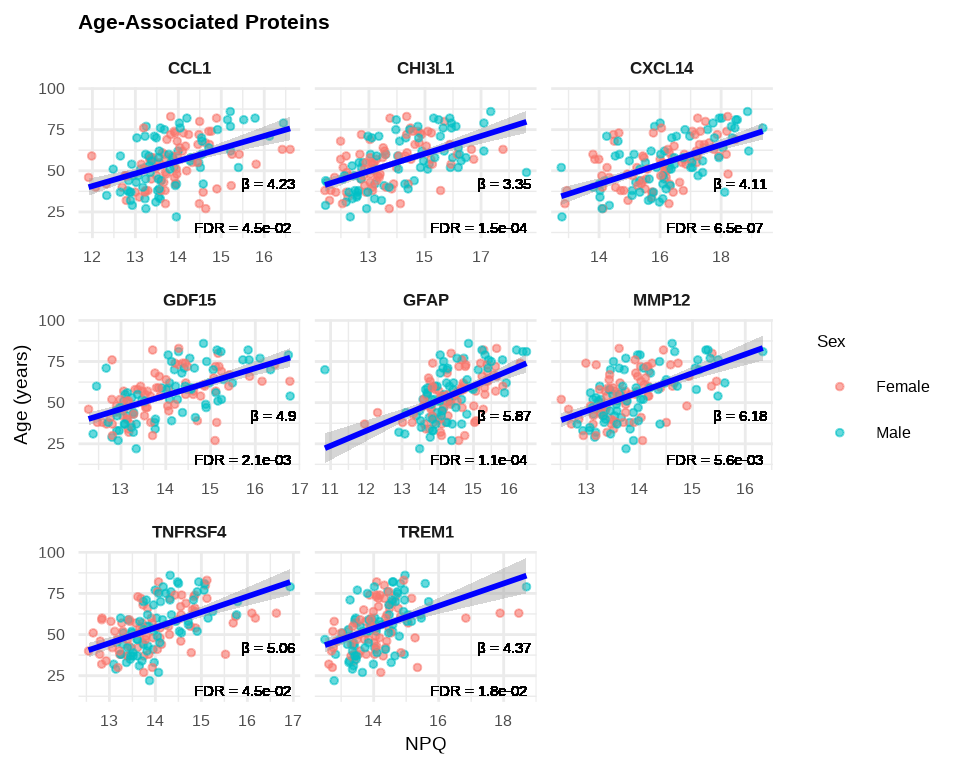
6.2.8 Age-Associated Proteins Scatterplots
# Create labels with available statistics
stat_labels <- lmpt_age$modelStats %>%
filter(target %in% age_proteins$target) %>%
select(target, target_coef, target_pval_FDR) %>%
mutate(label = paste0(
"β = ", round(target_coef, 2), "\n",
"FDR = ", format(target_pval_FDR, digits = 2, scientific = TRUE)
))
plot_data_age <- data_long %>%
filter(Target %in% age_proteins$target,
SampleName %in% sample_list_age) %>%
left_join(stat_labels, by = c("Target" = "target"))
# Plot age-associated proteins
ggplot(plot_data_age, aes(x = NPQ, y = age)) +
geom_point(aes(color = sex), alpha = 0.6, size = 1) +
geom_smooth(method = "lm", se = TRUE, color = "blue", linewidth = 1) +
geom_text(aes(label = label),
x = Inf, y = -Inf,
hjust = 1.1, vjust = -0.1,
size = 4, color = "black") +
facet_wrap(~ Target, scales = "free_x") +
theme_minimal() +
labs(title = "Age-Associated Proteins",
x = "NPQ",
y = "Age (years)",
color = "Sex") +
theme(
strip.text = element_text(size = 13, face = "bold"),
plot.title = element_text(size = 16, face = "bold"),
axis.title.x = element_text(size = 14),
axis.title.y = element_text(size = 14),
axis.text.x = element_text(size = 12),
axis.text.y = element_text(size = 12),
legend.title = element_text(size = 13),
legend.text = element_text(size = 12)
)
6.3 Example 2: Modeling Disease Status (Binary Outcome)
Now we will use logistic regression to model the association between protein abundance and disease status (inflammatory disease vs normal), adjusting for age and sex.
6.3.1 Disease Status as Binary Outcome
Question: Which proteins are associated with inflammatory disease after accounting for age and sex?
Unlike differential abundance which compares group mean NPQ levels, logistic regression models the relationship between continuous protein abundance and a binary outcome (disease present/absent) while adjusting for other factors.
6.3.2 Preparing the Disease Data
# Subset metadata for inflammatory disease and normal samples only
metadata_disease <- metadata %>%
filter(disease_type %in% c("normal", "inflam")) %>%
mutate(disease_binary = ifelse(disease_type == "inflam", 1, 0))
# Check outcome distribution
cat("Sample distribution:\n")#> Sample distribution:#>
#> 0 1
#> normal 79 0
#> inflam 0 21
#> cancer 0 0
#> kidney 0 0
#> metab 0 0
#> neuro 0 06.3.3 Building the Disease Association Model
# Fit logistic regression models for ALL proteins
glmt_disease <- glmNULISAseq_predict(
data = data$merged$Data_NPQ[, disease_sample_list],
sampleInfo = metadata_disease,
sampleName_var = "SampleName",
response_var = "disease_binary",
modelFormula = "sex + age" # Adjust for sex and age as covariates
)6.3.4 Understanding the Disease Model
For each protein, the model is:
log(odds of inflammatory disease) ~ NPQ + sex + ageThis tests: “Is this protein associated with disease status (inflammatory disease vs normal) while accounting for sex and age?”
6.3.5 Examining Disease Association Results
#> [1] "modelStats"#> Preview of Disease Association Results (rounded to 3 digits):Key Columns in Results
target: Protein nametarget_coef: Log odds ratio (raw coefficient)target_OR: Odds ratio = exp(target_coef)target_se: Standard error of the coefficienttarget_zval: Z-value (Wald statistic)target_pval_unadj: Unadjusted p-value (Wald test)target_pval_FDR: FDR-adjusted p-value
6.3.6 Finding Disease-Associated Proteins
We will identify proteins that are significantly associated with the odds of inflammatory disease with FDR-adjusted p value < 0.05.
# Find proteins significantly associated with disease status
disease_proteins <- glmt_disease$modelStats %>%
filter(target_pval_FDR < 0.05) %>%
arrange(target_pval_FDR) %>%
select(target, target_coef, target_OR, target_pval_FDR)
cat("Proteins significantly associated with disease status (FDR < 0.05):",
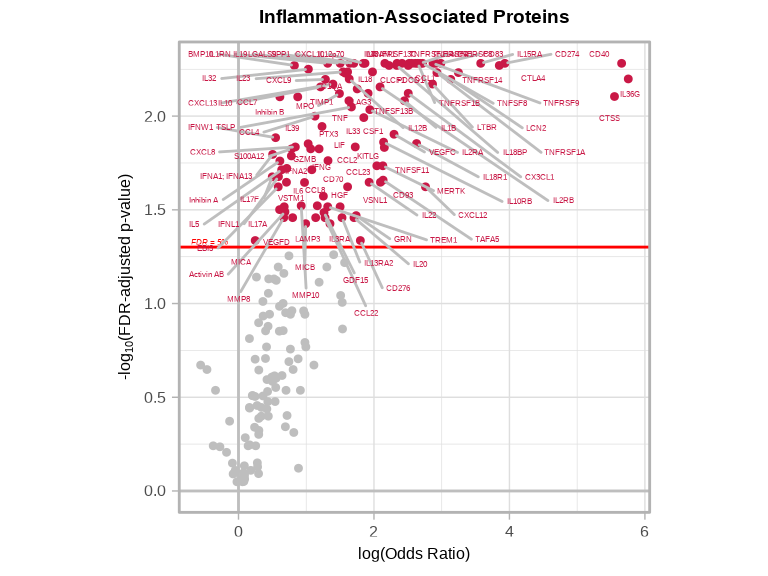
nrow(disease_proteins), "\n")#> Proteins significantly associated with disease status (FDR < 0.05): 1056.3.7 Interpreting Odds Ratios
# Strong positive associations with inflammatory disease (OR > 1)
inflam_associated <- disease_proteins %>%
filter(target_OR > 1) %>%
arrange(desc(target_OR))
cat("Number of proteins with significantly POSITIVE association with inflammatory disease:", nrow(inflam_associated), "\n\n")#> Number of proteins with significantly POSITIVE association with inflammatory disease: 105# Strong negative associations with inflammatory disease (OR < 1)
normal_associated <- disease_proteins %>%
filter(target_OR < 1) %>%
arrange(target_OR)
cat("Number of proteins with significantly NEGATIVE association with inflammatory disease:", nrow(normal_associated), "\n\n")#> Number of proteins with significantly NEGATIVE association with inflammatory disease: 0
Odds Ratio Interpretation:
- OR = 1: No association with disease status
- OR = 2: Each 1-unit increase in protein abundance (NPQ) is associated with 2 times higher odds of inflammatory disease
- OR = 0.5: Each 1-unit increase in protein abundance is associated with half the odds of inflammatory disease
- OR > 2: Strong positive association (higher protein → greater odds of inflammatory disease)
- OR < 0.5: Strong negative association (higher protein → lower odds of inflammatory disease)
6.3.8 Disease-Associated Proteins Volcano Plots
volcanoPlot(
coefs = glmt_disease$modelStats$target_coef,
p_vals = glmt_disease$modelStats$target_pval_FDR,
target_labels = glmt_disease$modelStats$target,
title = "Inflammation-Associated Proteins",
xlab = expression("log(Odds Ratio)")
)
6.3.9 Visualizing Disease Associations
# Plot top 6 disease-predictive proteins
top_6_disease <- disease_proteins$target[1:6]
# Box plots
data_long %>%
filter(Target %in% top_6_disease, SampleName %in% inflam_samples) %>%
ggplot(aes(x = disease_type, y = NPQ, fill = disease_type)) +
geom_boxplot(alpha = 0.7, outlier.shape = NA) +
geom_jitter(width = 0.2, alpha = 0.4, size = 1.5) +
facet_wrap(~ Target, scales = "free_y", ncol = 3) +
scale_fill_manual(values = c("normal" = "#4DAF4A", "inflam" = "#E41A1C")) +
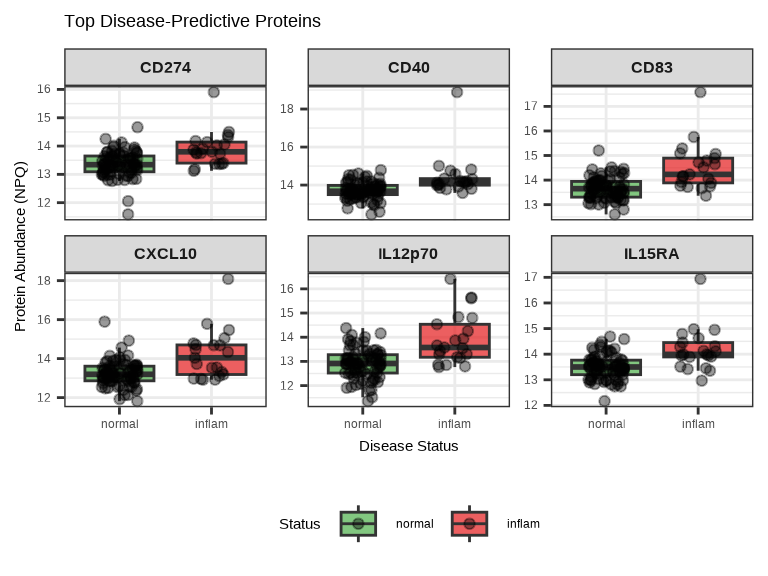
labs(title = "Top Disease-Predictive Proteins",
x = "Disease Status",
y = "Protein Abundance (NPQ)",
fill = "Status") +
theme(
strip.text = element_text(size = 12, face = "bold"),
legend.position = "bottom"
)
# Logistic curve visualization for top 6 proteins
data_long %>%
filter(Target %in% top_6_disease, SampleName %in% inflam_samples) %>%
left_join(
metadata_disease %>% select(SampleName, disease_binary),
by = "SampleName"
) %>%
ggplot(aes(x = NPQ, y = disease_binary)) +
geom_point(alpha = 0.5, size = 2) +
geom_smooth(method = "glm", method.args = list(family = "binomial"),
se = TRUE, color = "red", linewidth = 1) +
facet_wrap(~ Target, scales = "free_x", ncol = 3) +
theme_minimal() +
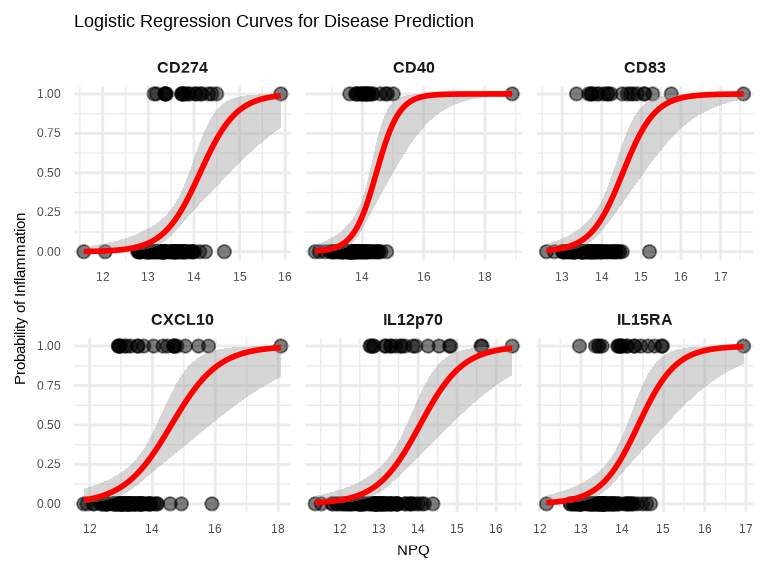
labs(title = "Logistic Regression Curves for Disease Prediction",
x = "NPQ",
y = "Probability of Inflammation") +
theme(
strip.text = element_text(size = 12, face = "bold")
)
6.3.10 ROC Curves for Disease-Associated Proteins
library(pROC)
# Prepare data for ROC analysis
roc_data <- data_long %>%
filter(Target %in% top_6_disease, SampleName %in% disease_sample_list) %>%
left_join(metadata_disease %>% select(SampleName, disease_binary),
by = "SampleName")
# Calculate ROC curves and AUC for each protein
roc_plot_data <- do.call(rbind, lapply(top_6_disease, function(protein) {
# Filter data for current protein
protein_data <- roc_data %>% filter(Target == protein)
# Calculate ROC
roc_obj <- roc(protein_data$disease_binary, protein_data$NPQ, quiet = TRUE)
# Extract ROC data and AUC
data.frame(
Target = protein,
Sensitivity = roc_obj$sensitivities,
Specificity = roc_obj$specificities,
AUC = round(as.numeric(auc(roc_obj)), 3)
)
}))
# Create annotation data
annotation_data <- unique(roc_plot_data[, c("Target", "AUC")])
annotation_data$label <- paste0("AUC = ", annotation_data$AUC)
# Create ROC curve plot
ggplot(roc_plot_data, aes(x = 1 - Specificity, y = Sensitivity)) +
geom_line(color = "#E41A1C", linewidth = 1) +
geom_abline(slope = 1, intercept = 0, linetype = "dashed", color = "gray50") +
geom_text(data = annotation_data,
aes(x = 0.7, y = 0.15, label = label),
size = 3.5, hjust = 0.1) +
facet_wrap(~ Target, ncol = 3) +
theme_minimal() +
labs(title = "ROC Curves for Top Disease-Associated Proteins",
x = "1 - Specificity (False Positive Rate)",
y = "Sensitivity (True Positive Rate)") +
theme(
strip.text = element_text(size = 13, face = "bold"),
plot.title = element_text(size = 14, face = "bold"),
axis.title = element_text(size = 12),
axis.text = element_text(size = 10)
) +
coord_equal()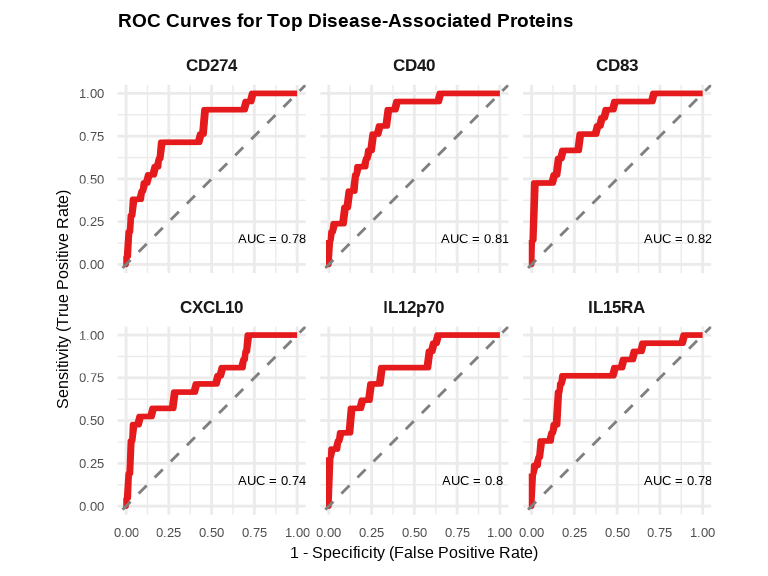
6.4 Comparing Approaches
6.4.1 When to Use Each Function
Differential Abundance (lmNULISAseq)
- Goal: Find proteins that show difference in mean abundance levels between groups
- Protein abundances (NPQ) are outcomes, groups are predictors
- Example: “Which proteins show different levels in inflammatory disease vs normal?”
Continuous Outcome (lmNULISAseq_predict)
- Goal: Identify proteins associated with a continuous outcome such as age or clinical score
- Proteins are predictors, continuous variable is outcome
- Example: “Which proteins can explain the most variation in age or disease severity score?”
Binary Outcome (glmNULISAseq_predict)
- Goal: Identify proteins associated with a binary outcome
- Proteins are predictors, binary variable is outcome
- Example: “Which proteins are most discriminative when classifying subjects into binary disease categories?”
6.5 Exporting Results
Save your results for downstream analysis or reporting:
# Save age association results
write_csv(lmpt_age$modelStats, "results/age_association_all_proteins.csv")
write_csv(age_proteins, "results/age_associated_proteins_significant.csv")
# Save disease association results
write_csv(glmt_disease$modelStats, "results/disease_association_all_proteins.csv")
write_csv(disease_proteins, "results/disease_associated_proteins_significant.csv")6.6 Complete Outcome Modeling Workflow
Here’s a complete workflow example that you can adapt for your own data:
# Load libraries
library(NULISAseqR)
library(tidyverse)
library(pROC)
# 1. Continuous Outcome: Age Association
## Check age distribution in the Detectability Study dataset used in differential abundance analysis
summary(metadata$age)
hist(metadata$age, main = "Age Distribution", xlab = "Age (years)")
# Exclude samples with missing age
metadata_age <- metadata %>% filter(!is.na(age))
sample_list_age <- metadata_age$SampleName
## Fit linear models
lmpt_age <- lmNULISAseq_predict(
data = data$merged$Data_NPQ[, sample_list_age],
sampleInfo = metadata_age,
sampleName_var = "SampleName",
response_var = "age",
modelFormula = "disease_type + sex"
)
## Identify significant age-associated proteins
age_proteins <- lmpt_age$modelStats %>%
filter(target_pval_FDR < 0.05) %>%
arrange(target_pval_FDR)
cat("Proteins associated with age:", nrow(age_proteins), "\n\n")
## Volcano plot for age-associated proteins
volcanoPlot(
coefs = lmpt_age$modelStats$target_coef,
p_vals = lmpt_age$modelStats$target_pval_FDR,
target_labels = lmpt_age$modelStats$target,
title = "Age-Associated Proteins",
xlab = "Coefficient (Years of age per unit NPQ)",
)
## Plot age-associated proteins
stat_labels <- lmpt_age$modelStats %>%
filter(target %in% age_proteins$target) %>%
select(target, target_coef, target_pval_FDR) %>%
mutate(label = paste0(
"β = ", round(target_coef, 2), "\n",
"FDR = ", format(target_pval_FDR, digits = 2, scientific = TRUE)
))
plot_data_age <- data_long %>%
filter(Target %in% age_proteins$target,
SampleName %in% sample_list_age) %>%
left_join(stat_labels, by = c("Target" = "target"))
# Plot age-associated proteins
ggplot(plot_data_age, aes(x = NPQ, y = age)) +
geom_point(aes(color = sex), alpha = 0.6, size = 1) +
geom_smooth(method = "lm", se = TRUE, color = "blue", linewidth = 1) +
geom_text(aes(label = label),
x = Inf, y = -Inf,
hjust = 1.1, vjust = -0.1,
size = 4, color = "black") +
facet_wrap(~ Target, scales = "free_x") +
theme_minimal() +
labs(title = "Age-Associated Proteins",
x = "NPQ",
y = "Age (years)",
color = "Sex") +
theme(
strip.text = element_text(size = 13, face = "bold"),
plot.title = element_text(size = 16, face = "bold"),
axis.title.x = element_text(size = 14),
axis.title.y = element_text(size = 14),
axis.text.x = element_text(size = 12),
axis.text.y = element_text(size = 12),
legend.title = element_text(size = 13),
legend.text = element_text(size = 12)
)
# 2. Binary Outcome: Disease Status
## Prepare binary outcome
metadata_disease <- metadata %>%
filter(disease_type %in% c("normal", "inflam")) %>%
mutate(disease_binary = ifelse(disease_type == "inflam", 1, 0))
disease_sample_list <- metadata_disease$SampleName
## Fit logistic regression models
glmt_disease <- glmNULISAseq_predict(
data = data$merged$Data_NPQ[, disease_sample_list],
sampleInfo = metadata_disease,
sampleName_var = "SampleName",
response_var = "disease_binary",
modelFormula = "sex + age"
)
## Identify significant associations
disease_proteins <- glmt_disease$modelStats %>%
filter(target_pval_FDR < 0.05) %>%
arrange(target_pval_FDR)
cat("Proteins associated with disease:", nrow(disease_proteins), "\n\n")
## Identify proteins with positive associations with inflammatory disease (OR > 1)
inflam_associated <- disease_proteins %>%
filter(target_OR > 1) %>%
arrange(desc(target_OR))
cat("Number of proteins with significantly POSITIVE association with inflammatory disease:", nrow(inflam_associated), "\n\n")
# Identify proteins with negative associations with inflammatory disease (OR < 1)
normal_associated <- disease_proteins %>%
filter(target_OR < 1) %>%
arrange(target_OR)
cat("Number of proteins with significantly NEGATIVE association with inflammatory disease:", nrow(normal_associated), "\n\n")
## Volcano plot for disease-associated proteins
volcanoPlot(
coefs = glmt_disease$modelStats$target_coef,
p_vals = glmt_disease$modelStats$target_pval_FDR,
target_labels = glmt_disease$modelStats$target,
title = "Inflammation-Associated Proteins",
xlab = expression("log"[2] * "(Odds Ratio)")
)
# 3. Visualize GLM predictions for top proteins
## Top 6 disease-predictive proteins
top_6_disease <- disease_proteins$target[1:6]
## Boxplots visualization for top 6 proteins
data_long %>%
filter(Target %in% top_6_disease, SampleName %in% inflam_samples) %>%
ggplot(aes(x = disease_type, y = NPQ, fill = disease_type)) +
geom_boxplot(alpha = 0.7, outlier.shape = NA) +
geom_jitter(width = 0.2, alpha = 0.4, size = 1.5) +
facet_wrap(~ Target, scales = "free_y", ncol = 3) +
scale_fill_manual(values = c("normal" = "#4DAF4A", "inflam" = "#E41A1C")) +
labs(title = "Top Disease-Predictive Proteins",
x = "Disease Status",
y = "Protein Abundance (NPQ)",
fill = "Status") +
theme(
strip.text = element_text(face = "bold"),
legend.position = "bottom"
)
## Logistic curve visualization for top 6 proteins
data_long %>%
filter(Target %in% top_6_disease, SampleName %in% inflam_samples) %>%
left_join(
metadata_disease %>% select(SampleName, disease_binary),
by = "SampleName"
) %>%
ggplot(aes(x = NPQ, y = disease_binary)) +
geom_point(alpha = 0.5, size = 2) +
geom_smooth(method = "glm", method.args = list(family = "binomial"),
se = TRUE, color = "red", linewidth = 1) +
facet_wrap(~ Target, scales = "free_x", ncol = 3) +
theme_minimal() +
labs(title = "Logistic Regression Curves for Disease Prediction",
x = "NPQ",
y = "Probability of Inflammation") +
theme(
strip.text = element_text(size = 10, face = "bold")
)
## ROC Curves for top 6 proteins
# Prepare data for ROC analysis
roc_data <- data_long %>%
filter(Target %in% top_6_disease, SampleName %in% disease_sample_list) %>%
left_join(metadata_disease %>% select(SampleName, disease_binary),
by = "SampleName")
roc_plot_data <- do.call(rbind, lapply(top_6_disease, function(protein) {
# Filter data for current protein
protein_data <- roc_data %>% filter(Target == protein)
# Calculate ROC
roc_obj <- roc(protein_data$disease_binary, protein_data$NPQ, quiet = TRUE)
# Extract ROC data and AUC
data.frame(
Target = protein,
Sensitivity = roc_obj$sensitivities,
Specificity = roc_obj$specificities,
AUC = round(as.numeric(auc(roc_obj)), 3)
)
}))
annotation_data <- unique(roc_plot_data[, c("Target", "AUC")])
annotation_data$label <- paste0("AUC = ", annotation_data$AUC)
## Plot ROC curves
ggplot(roc_plot_data, aes(x = 1 - Specificity, y = Sensitivity)) +
geom_line(color = "#E41A1C", linewidth = 1) +
geom_abline(slope = 1, intercept = 0, linetype = "dashed", color = "gray50") +
geom_text(data = annotation_data,
aes(x = 0.7, y = 0.15, label = label),
size = 3.5, hjust = 0.1) +
facet_wrap(~ Target, ncol = 3) +
theme_minimal() +
labs(title = "ROC Curves for Top Disease-Associated Proteins",
x = "1 - Specificity (False Positive Rate)",
y = "Sensitivity (True Positive Rate)") +
theme(
strip.text = element_text(size = 13, face = "bold"),
plot.title = element_text(size = 14, face = "bold"),
axis.title = element_text(size = 12),
axis.text = element_text(size = 10)
) +
coord_equal()
# 4. Exports all results
dir.create("results", showWarnings = FALSE)
write_csv(age_proteins, "results/age_associated_proteins.csv")
write_csv(disease_proteins, "results/disease_associated_proteins.csv")
cat("Results exported successfully!\n\n")
# 6. Summary
cat("Age-associated proteins:", nrow(age_proteins), "\n")
cat("Disease-associated proteins:", nrow(disease_proteins), "\n")6.7 Best Practices
Model Design
Covariate Selection
- Include biologically relevant covariates (e.g., age, sex)
- Include covariates that capture technical variation when needed (e.g., batch)
- Avoid overfitting: don’t include too many covariates relative to sample size
- Check for multicollinearity (i.e., correlation) between covariates
Model Considerations
- For continuous outcomes: Check for non-linear relationships (consider polynomial terms if needed)
- For binary outcomes: Ensure adequate events per variable (typically ≥10 events per covariate)
- Test for interactions if biologically plausible (e.g., protein × age interaction)
Interpretation
Statistical Significance vs. Biological Relevance
- FDR < 0.05 indicates false-discovery rate corrected statistical significance
- Effect size matters: small p-values don’t always mean biological importance
- For age: Consider whether coefficient magnitude is biologically meaningful
- For disease: OR > 2 or OR < 0.5 typically indicate strong associations
Model Assessment
- For linear models: Check residual plots for normality and homoscedasticity
- For logistic models: Large coefficients or OR near 0/infinity may indicate separation issues
- Compare multiple proteins: consistency across related proteins strengthens confidence
Understanding Differences Between Methods
- Differential abundance and logistic regression ask different questions - both provide valuable insights
- Proteins significant in both analyses are typically the most robust findings
- Method-specific findings warrant further investigation but may be true biological signals or in some cases driven by outliers
Validation
Within-Dataset Validation
- Examine sensitivity to covariate specification
- Check robustness across different subgroups
External Validation
- Validate findings in independent datasets when possible
- Compare with published literature
Reporting
Essential Information to Report
- Model formula with all covariates
- Sample sizes (total and by group)
- Number of proteins tested and significant
- Multiple testing correction method (FDR, Bonferroni)
Transparent Reporting
- Report both significant and non-significant results for key proteins of interest
- Acknowledge limitations (sample size, covariates not adjusted for, etc.)
- Clearly distinguish exploratory from confirmatory analyses
- Make data and code available when possible
Visualization Guidelines
- Show raw data points when possible (not just summary statistics)
- Include uncertainty estimates (SE, confidence intervals)
- Use consistent color schemes across figures
- Clearly label axes and provide informative legends
Continue to: Chapter 7: Case Study: Identifying Proteomic Signatures in SLE and RA